马上注册,结交更多好友,享用更多功能,让你轻松玩转社区。
您需要 登录 才可以下载或查看,没有账号?立即注册

x
上周末真是热闹非凡。AHA正在如火如荼地进行,阿斯利康的痛风药物lesinurad也公布了三期临床数据,新英格兰医学杂志又发表了两篇黑色素瘤的重要进展。今天就重点谈谈nivolumab
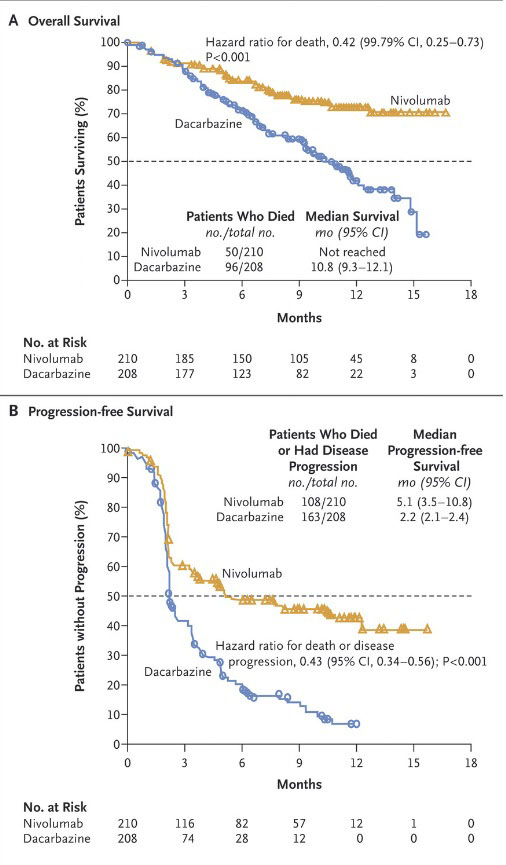
11.16日发表的就是著名的所谓checkmate-066实验结果,比较PD-1抑制剂nivolumab和传统化疗药物dacarbazine在未经治疗、无BRAF变异晚期黑色素瘤患者的疗效和安全性。这个实验由于疗效显著在今年6月提前结束,但今天正式发表了实验数据。二者的数据比较如下:一年生存率73%对42%;应答率40%对14%;无进展生存期5.1对2.2个月;3级以上副作用:11.7%对17.6%。这是PD-1抑制剂首次在三期临床实验中显示生存疗效,而且副作用低于化疗。下面这条Kaplan-Meier曲线相当震撼,对病人来说就是救命稻草,对厂家来说就是真金白银,对科学家来说这曲线就是一个艺术品。
PD-1抑制剂越来越出落成抗癌领域的明星。不仅彻底改变了晚期黑色素瘤的治疗,在非小细胞肺癌也达到了远高于其它疗法的应答,在其它实体瘤如膀胱癌、头颈癌也显示一定前景。原来认为黑色素瘤是个小适应症,但有人重新估计这个适应症是被低估了,可达50亿美元。肺癌是大得多的适应症,据估计PD-1市场总值可达350亿美元。但这些估计是在生存优势未知的情况下做出的,如果PD-1抑制剂能像黑色素瘤一样显著延长肺癌患者的生命,那么病人数量会迅速累积,市场总额也会随之增加。
source: http://yypharm.cn/news/kzll/2014-11-16/882.html |
|
|
|
|
|
|
共1条精彩回复,最后回复于 2014-11-18 12:22

尚未签到
Nivolumab in Previously Untreated Melanoma without BRAF Mutation
BACKGROUND
Nivolumab was associated with higher rates of objective response than chemotherapy in a phase 3 study involving patients with ipilimumab-refractory metastatic melanoma. The use of nivolumab in previously untreated patients with advanced melanoma has not been tested in a phase 3 controlled study.
METHODS
We randomly assigned 418 previously untreated patients who had metastatic melanoma without a BRAF mutation to receive nivolumab (at a dose of 3 mg per kilogram of body weight every 2 weeks and dacarbazine-matched placebo every 3 weeks) or dacarbazine (at a dose of 1000 mg per square meter of body-surface area every 3 weeks and nivolumab-matched placebo every 2 weeks). The primary end point was overall survival.
RESULTS
At 1 year, the overall rate of survival was 72.9% (95% confidence interval [CI], 65.5 to 78.9) in the nivolumab group, as compared with 42.1% (95% CI, 33.0 to 50.9) in the dacarbazine group (hazard ratio for death, 0.42; 99.79% CI, 0.25 to 0.73; P<0.001). The median progression-free survival was 5.1 months in the nivolumab group versus 2.2 months in the dacarbazine group (hazard ratio for death or progression of disease, 0.43; 95% CI, 0.34 to 0.56; P<0.001). The objective response rate was 40.0% (95% CI, 33.3 to 47.0) in the nivolumab group versus 13.9% (95% CI, 9.5 to 19.4) in the dacarbazine group (odds ratio, 4.06; P<0.001). The survival benefit with nivolumab versus dacarbazine was observed across prespecified subgroups, including subgroups defined by status regarding the programmed death ligand 1 (PD-L1). Common adverse events associated with nivolumab included fatigue, pruritus, and nausea. Drugrelated adverse events of grade 3 or 4 occurred in 11.7% of the patients treated with nivolumab and 17.6% of those treated with dacarbazine.
CONCLUSIONS
Nivolumab was associated with significant improvements in overall survival and progression-free survival, as compared with dacarbazine, among previously untreated patients who had metastatic melanoma without a BRAF mutation. (Funded by Bristol-Myers Squibb; CheckMate 066 ClinicalTrials.gov number, NCT01721772.)
 nejmoa1412082.pdf
(2.68 MB, 下载次数: 67)
nejmoa1412082.pdf
(2.68 MB, 下载次数: 67)
|
|
|
|
|
|
|